EU-GMP: The Gateway for Vietnamese Pharmaceuticals to the World – Strategic Partnership Opportunity with UIP
Why is EU-GMP the “Golden Ticket” for Vietnamese Pharmaceuticals?
As global pharmaceutical regulations become increasingly stringent, EU-GMP (European Good Manufacturing Practice) is not just a certification — it’s a “golden passport” to global markets. The path to international integration is now more accessible, especially when partnering with UIP – a strategic and trusted partner with over 30 years of leadership in Vietnam’s pharmaceutical industry.
In January 2025, UIP proudly obtained EU-GMP certification. EU-GMP is a rigorous set of rules and guidelines issued by the European Union to ensure all pharmaceutical products are manufactured and quality-controlled to the highest international standards.
Once a product is EU-GMP certified, it gains several critical advantages:
- Global Market Access: Certified products can be registered and distributed in highly regulated markets that prioritize quality and safety.
- Enhanced Brand Credibility: A strong declaration of product quality and reliability, increasing potential for international partnerships and consumer trust.
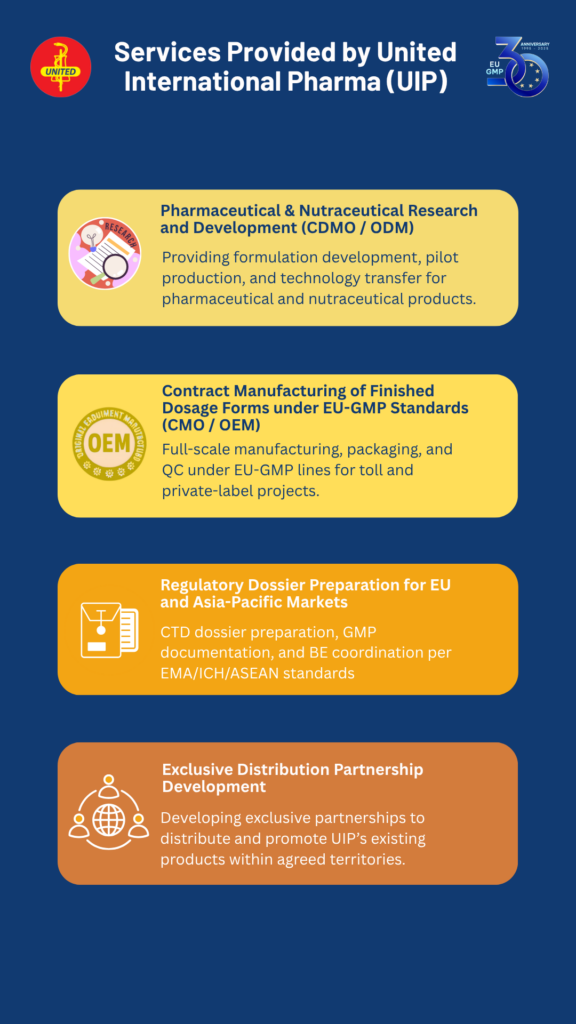
Services Provided by United International Pharma (UIP):
- New Product Development (NPD): Pharmaceutical & Nutraceutical Research and Development (CDMO / ODM)
- Tolling: Contract Manufacturing of Finished Dosage Forms under EU-GMP Standards (CMO / OEM)
- Export: Regulatory Dossier Preparation for EU and Asia-Pacific Markets
- Outsourcing: Exclusive Distribution Partnership Development
Why Choose UIP?
- State-of-the-art Technology & Infrastructure: Facilities strictly adhering to EU-GMP standards, ensuring international-grade production quality.
- Expert R&D Team from Unilab: From initial ideas to final product registration, our experienced R&D team supports every step of your journey.
- Comprehensive Quality Control: Premium systems with stringent controls from raw materials to finished products.
- Global (80+ years) & Local (30+ years) Experience: With deep understanding of regulatory processes, UIP helps reduce time-to-market and costs.
A Golden Opportunity for Vietnamese Pharma Enterprises
Partnering with UIP offers distinct advantages to help Vietnamese pharmaceutical companies achieve their export ambitions faster than ever.
Leverage “Made in Vietnam, International Standards”:
- Cost Optimization
- Faster Product Launch
- Stronger Market Competitiveness
- Access to High-Potential Markets
High-Potential Product Segments: EU-GMP Generic Drugs (ETC / OTC)
Don’t miss this opportunity. Contact UIP today to explore strategic collaboration and build a prosperous future together!